
Subscribe to our Telegram channels to get the latest job updates & much more.
Not on Telegram? We also send regular job updates on
Note: Would recommend subscribing to more than one channel. You need to click on Join/Subscribe/Follow after visiting the relevant channels.
Steps to get Job Updates & Much More in your Email Inbox !
- Click on theContinue to Google Groupbutton at the bottom of this popup.
- Click on the button as shown in the image below.Click on the button as shown in the image below.
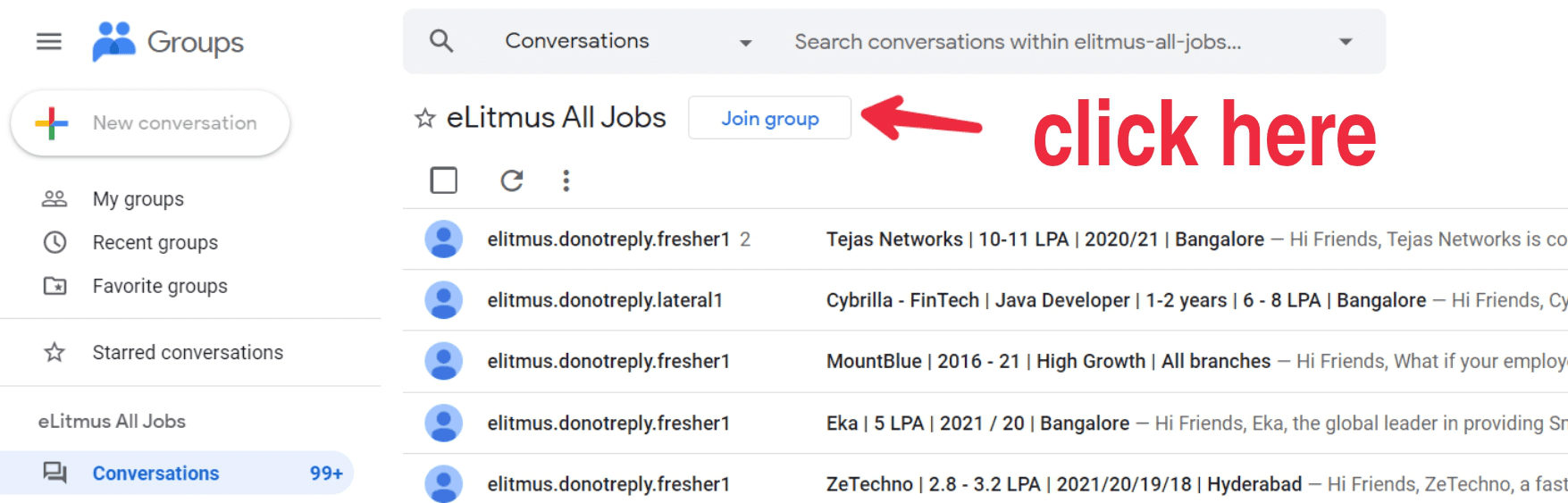
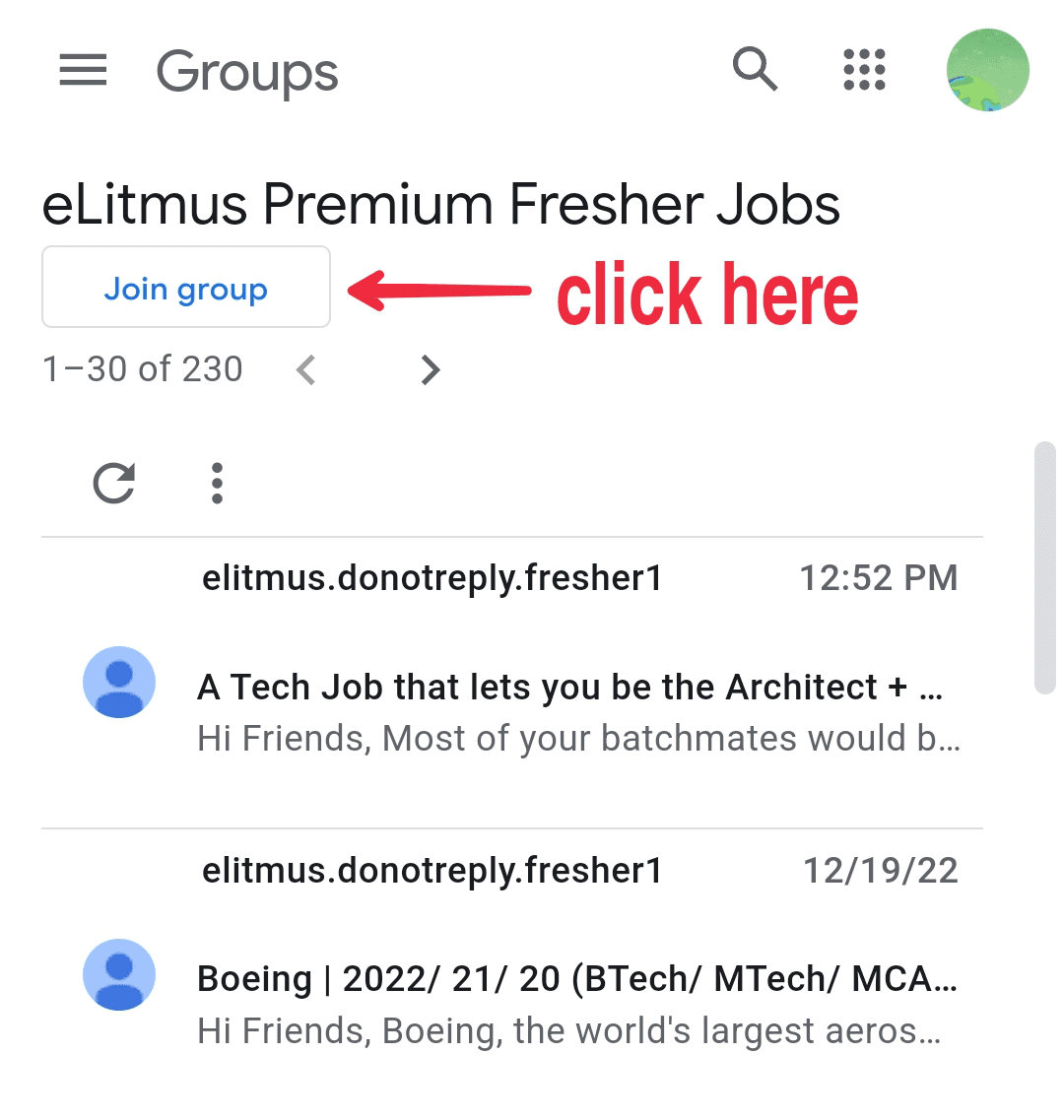
- Click on the button as shown in the image below.
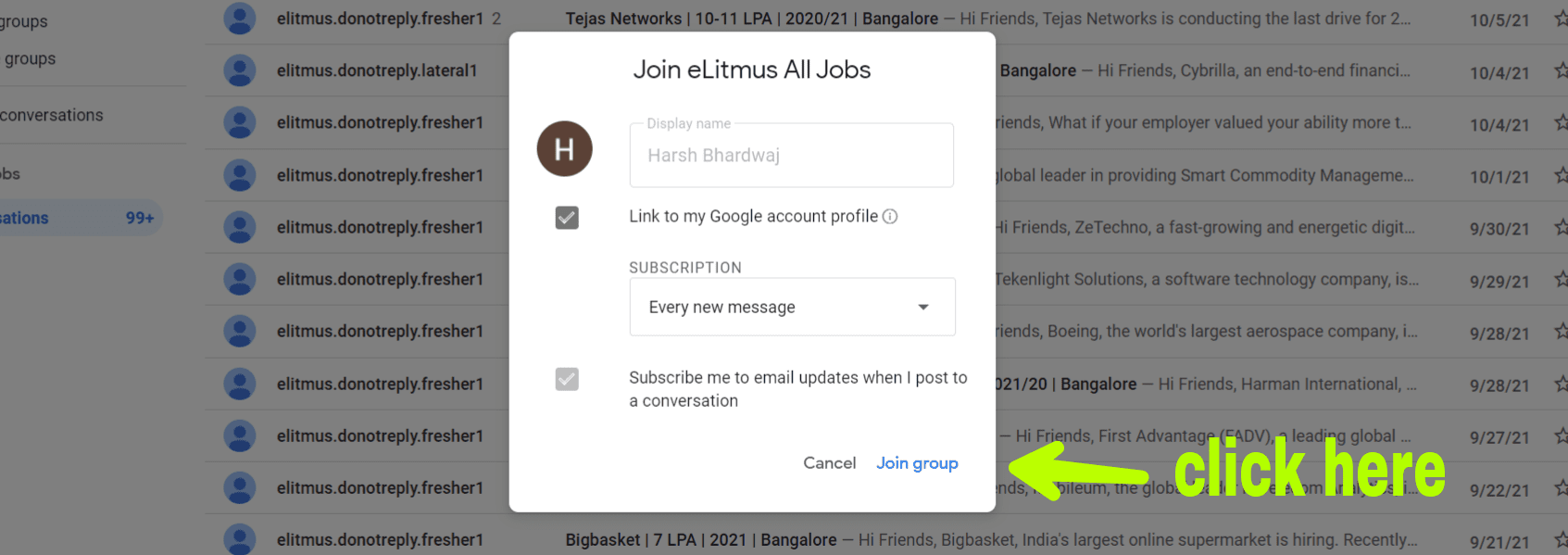
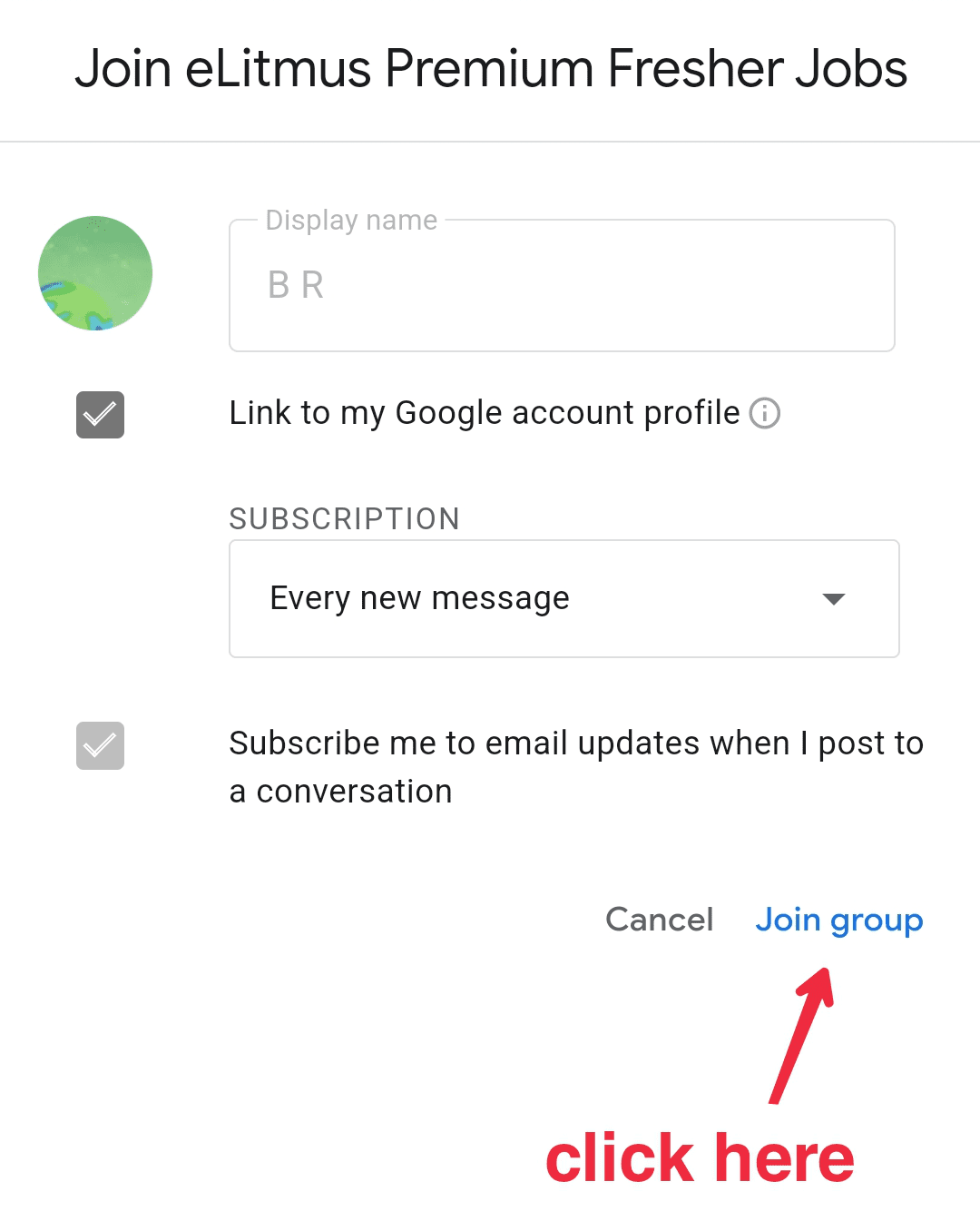

Dr Reddys Laboratories Ltd
www.drreddys.com
Full Time
Hyderabad, Vizag, Miryalguda
₹ 7,08,000
Fresher
About Company:
Dr. Reddy’s Laboratories Ltd. (NYSE: RDY) is an integrated global pharmaceutical company, committed to accelerating access to affordable and innovative medicines with a belief that Good Health Can’t Wait. Through its three businesses - Pharmaceutical Services and Active Ingredients, Global Generics, and Proprietary Products – and with 20,000+ associates worldwide Dr. Reddy’s offers a portfolio of products and services including APIs, custom pharmaceutical services, generics, biosimilars, differentiated formulations and NCEs. Therapeutic focus is on gastrointestinal, cardiovascular, diabetology, oncology, pain management, anti-infective and pediatrics. Major markets include India, USA, Russia and CIS, Germany, UK, S. Africa, Romania, and Australia.For more information log on to: www.drreddys.com
Eligibility:
- 2016 batch BE/ BTech (Chemical/Instrumentation)
- Good Academics
- Good pH score
- You should be from one of the following colleges
NITs - (Allahabad / Bhopal / Calicut / Durgapur / Hamirpur / Jalandhar / Jaipur / Jamshedpur / Kurukshetra / Nagpur / Raipur / Rourkela / Surat / Suratkal / Tiruchirapalli / Warangal)
BITS Pilani - (Pilani Campus only)
Job Description:
It will include one or more of the following
- Collate information & co-ordinate for preparation of project proposal with the inputs provided by end user such as capacity required and future expansion possibilities in next 3-5yrs.
- Prepare proposed location layout, scope document, basic process flow chart, project timelines and estimated budget.
- Review technical specifications and comparisons for equipment & materials w.r.t approved drawings and specifications in order to ensure monitoring as per defined project specification.
- Based on production target identify suitable batch size & equipment in order to meet production requirement as per the projections.
- Identify and implement methods for improvement in yield and quality, reduce product failures and achieve/decrease the budgetary cost for the given product.
- Identify processes wherein the utilities (Cooling tower water, Brine, Steam, Electricity) consumption can be optimized; suggest and implement suitable measures to reduce the cost of production and reduction of wastage.
- Will be responsible for understanding and operating Steam Sterilizers, Lyophilisers, Fluid Bed coaters, Fluid Bed Driers, Tablet coating machines.
- Will be responsible for understanding and operation of Automated Container Inspection machines, Automated tablet packing lines, blister packing lines, cartoners, end of line automated equipment in packing, ASRS systems and Automated Guided Vehicles (AGV’s) being used in Warehousing.
- Will need to understand and qualify equipment procured newly in Design Q, Installation Q, Operaional Q, Performance Q.
- Help in preparing ‘Recipes’ for processes involving Processing, Safety and Engineering Control.
- Understand and Participate in Electronic Batch Manufacturing Record (EBR), Packing Records.
- Understand and Participate in Data Acquisition systems (DAS) for integrating Process Equipment with EBR.
- Understand and Participate in Process Analytical Technology (PAT), Multivariate analysis in order to ensure robust processing activities.


